The GLC-MS is performed using a comercial low-resolution instrument. For a description of the design and function of such instruments the reader is reffered to standard textbooks. On-line connection to a mini-computer, which allows rapid handling and normalisation of recorded spectra, is advantageous but not necessary. The spectra obtained from different instruments may vary slightly but this does not affect interpretations. The interface between the gas chromatograph and the mass spectrometer (the carrier gas separator) should be of high quality and give minimum loss of components and peak-broadening. Mass spectrometers with high capacity pumping systems, which allow the use of capillary columns without a carrier gas separator, are available and are to be preferred to other instruments. The interface is heated to about 25°C above the temperature of the gas chromatograph oven. The temperature of the ion-source should be kept below 250°C to minimize thermal destruction of the components. Spectra are recorded at an ionisation potential of 70 eV with scan speeds recommended by the instrument manufacturer for GLC-MS operation. It is advisable to record several spectra on each peak from the chromatogram in order to ensure that the peak is homogenous with respect to a certain compound.
The fragmentation pathways of partially methylated alditol acetates on electron impact have been studied in great detail using the deuterium labelling technique 4 5 6 7 . From the primary and secondary fragmentations elucidated in these studies, the substitution pattern of the partially methylated alditol acetates can be readily determined. MS of these compounds as well as other derivatives of carbohydrates have been reviewed 8 32 and therefore only a brief treatment is given here. A collection of reference spectra is given in Appendix B.
Stereoisomeric partially methylated alditol acetates give very similar mass spectra and it is consequently not possible to determine from the mass spectra if a component derives e.g. from glucose, galactose or mannose. The molecular ions are rarely observed but molecular weights may be determinedby addition of pairs of fragments. Primary fragments are generated by fission between carbon atoms in the chain. Fission between two methoxylated carbons (I) is more abundant than fission between one methoxylated and one acetoxylated carbon (II) which in turn is more abundant than fission between two acetoxylated carbons (III). In case I both fragments appear as strong ions. In case II, however, only the methoxylated fragment appears as a strong ion. In case III, finally, both ions are weak, except for alditol acetates and mono-O-methyl alditol acetates in which the methoxyl group occupies a terminal position. These routes are illustrated below in Fig. 2.
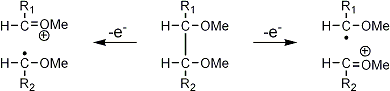 |
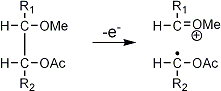 |
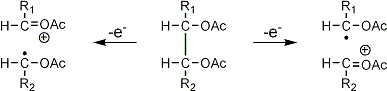 |
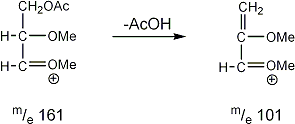 |
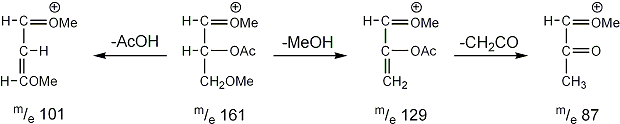 |
Some pairs of methylated sugars (e.g. 3- and 4-O-methylhexose) will, for symmetry reasons, give alditols with the same substitution pattern. This problem can be overcome by performing the reduction to alditols with sodium borodeuteride. Reduction with borodeuteride may also facilitate the interpretation of other mass spectra.
 Cite!
Cite!