Methylation analysis is the most facile method for determining the
substitution of the monosaccharide units constituting oligo- and polysaccharides
or the carbohydrate moieties of glycoconjugates. The method
gives details of the structural units present in the polymers, but
gives no information on their sequence or the anomeric natures of their
linkages. The analysis involves the conversion of all free hydroxyls
in the original material into methoxy groups, followed by hydrolytic
cleavage with acid of the glycosidic linkages and analysis of monomers
formed. The subsitution pattern of O-methyl groups in the monomers
reflects the substitution of the corresponding sugars in the native
material. The hydroxyl → methoxyl substitution in the native material is usually performed by the Hakomori procedure 1
(Sections 2.1 and 2.2)
and the analysis of the monomers formed on hydrolysis is carried out
by gas liquid chromatography (GLC) 2
3
and mass spectrometry (MS) of
the derived alditol acetates 4
5
6
7
(Sections 4 and 5). The steps involved
in methylation analysis by this method are illustrated in Fig. 1.
Methylation analysis has been reviewed earlier 8
9
and therefore
practical aspects will be emphasised in the following and the theoretical
discussion will be kept to a minimum.
Figure 1
Hypothetical native substrate
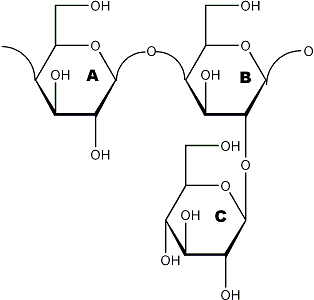 methylation →
methylation →
|
Methylated substrate
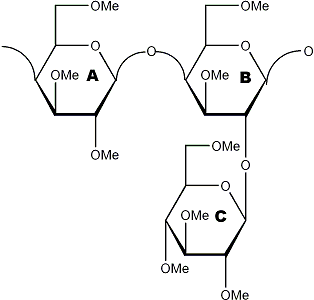 acid hydrolysis →
acid hydrolysis →
|
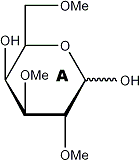
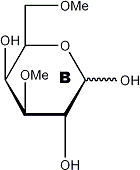
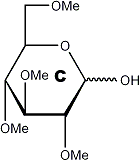
|
|
↓ 1) reduction 2) acetylation
|
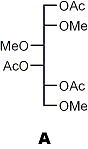
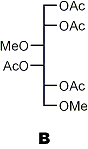
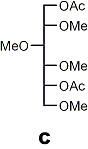
|
|
Partially methylated alditol acetates (analysis by GLC and MS)
|
|
|
 methylation →
methylation →
 acid hydrolysis →
acid hydrolysis →






 Cite!
Cite!