- Introduction
- Component analysis
- Linkage analysis
- Specific degradations
- Absolute configuration
- Purification
- Various procedures
- Appendix
- Search
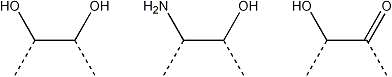
The following products are obtained from a terminal, a 2-substituted, a 4-substituted, a 6-substituted glucose residue after oxidation, reduction and mild acid hydrolysis.
| Terminal | 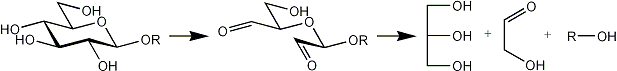 |
| 2-O-substituted | 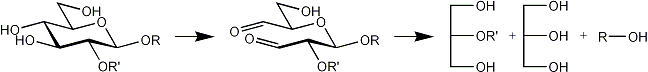 |
| 4-O-substituted | 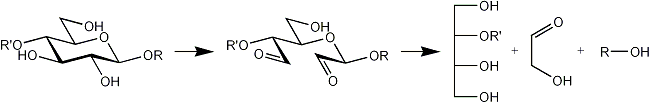 |
| 6-O-substituted | 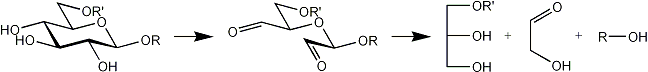 |
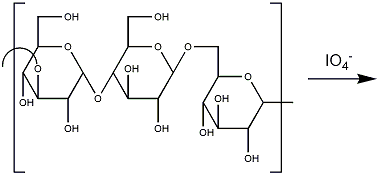
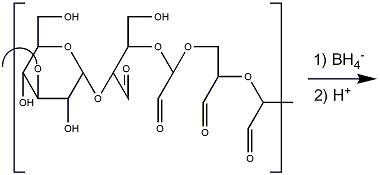
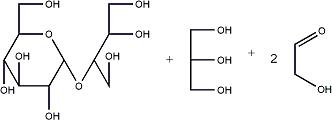
Reagents
- Acetate buffer 0.1M, pH 3.9
- NaIO4 , 0.03M
- Ethylene glycol
- HOAc (10%)
- NaBH4
- CF3COOH (TFA), 0.5M
- NaOH, 4M
- Dialysis tubing
Procedure
- Dissolve the polysaccharide (10-50 mg) in acetate buffer, ca 1 mg/mL
- Add NaIO4 to a final concentration of 0.03M, leave in the dark (Al foil) in a refrigerator (4°C) for 72h.
- Destroy excess NaIO4 with ethylene glycol (100 µL per 10 µL buffer solution) leave for 2 h and put in dialysis bag under running tap water for >6h. Concentrate if diluted too much.
- Reduce the oxidised product with NaBH4 (3 mg per mg polysaccharide), leave for 3h, make pH 7 with 10% aqueous HOAc, dialyse under tap water and then distilled water. Evaporate to a small volume.
- Optional: check by methylation analysis that all periodate susceptible residues are oxidised. If not, do the oxidation-reduction sequence once more.
- Make the solution 0.5M with respect to CF3CO2H by adding the appropriate volume of acid. Leave for 24h at room temperature.
- Evaporate most of the acid, and make pH 7 by NaOH, purify by chromatography on Bio-Rad P2
Comments
- The buffer and the dark is for avoiding overoxidation, i.e. only aldehydes should be formed and no acids. The consumption of NaIO4 could be followed, when no more reagent is consumed you could proceed.
- The periodate is reduced to iodate and the ethylene glycol is cleaved to form formaldehyde which is removed by dialysis not to consume NaBH4 .
- The aldehyde groups in the oxidised residues are now reduced to alcohols.
- Sometimes not all diols can be oxidised because carbonyl groups from vicinal residues react with one of the hydroxyls to form hemiacetals. This hemiacetal is opened in the reduction step and the diol regenerated. A second cycle of oxidation-reduction must then be performed.
- The hydrolysis step is performed at room temperature because at higher temperatures the differences in hydrolysis rate between cyclic and acyclic acetals is smaller. Lowering the temperature would lead to unreasonable hydrolysis times.
- The resulting oligo- or polysaccharide is isolated and analysed. Often fairly poor yields of product are obtained. Sometimes by-products with a ring structure at the "reducing" end is obtained instead of an alditol. If uronic acids are present and oxidised the hydrolysis of such modified residues is often slow.
References
- G.W. Hay, B.A. Lewis and F. Smith, Methods Carbohydr. Chem. V (1965) 357-361.
- I.J. Goldstein, G.W. Hay, B.A. Lewis and F. Smith, Methods Carbohydr. Chem. V (1965) 361-370.